Background: High-dose melphalan (MEL) followed by autologous hematopoietic stem cell transplantation (AHSCT) remains the treatment of choice for newly diagnosed multiple myeloma (MM) patients who are transplant-eligible. Despite variations in response, AHSCT outcomes are crucial in MM patients. Complete remission (CR) correlates with extended overall survival, while early relapse is linked to reduced survival, irrespective of cytogenetic risk. Identifying predictive biomarkers that might stratify patients according to their chance of achieving a response to AHSCT is essential for optimizing maintenance and consolidation therapy and improving patient outcomes.
Aims: In our study, we investigated the changes in serum exosomal content in AHSCT recipients during the procedure and the potential differences in proteomic signatures depending on the response to AHSCT.
Materials and methods: The study group consisted of 27 MM (12 women, 15 men) patients. The mean age was 60.5 ± 8.0 years. Nine patients (33.3%) had stage III, according to the Revised International Staging System (R-ISS). Most patients (18, 66.7%) received VCD (bortezomib, cyclophosphamide, dexamethasone) as an induction regimen. Most patients (18, 66.7%) received a MEL-200 mg/m2 conditioning regimen, 9 (33.3%) received a MEL-140. Patients received a median of 4.2 x 10 6 CD34+ cells/kg. Response assessment was available in 26 patients (96.4%). At +100 days after AHSCT, 12 patients (44.4%) achieved CR, four patients (14.8%) had a very good partial response, 6 (22.2%) had a partial response (PR), two patients (7.4%) had stable disease and two patients had disease progression.
Blood samples were collected at four time points: before conditioning chemotherapy (T1), on day 0 (T2), day +7 (T3), and on day +14 after AHSCT (T4). Exosomes were isolated from 108 serum samples using exoEasy Maxi Kit (Qiagen). To perform protein identification, the proteins from exosome samples were trypsin digested. The obtained peptides were labelled using TMT10plex™ Isobaric Label Reagent Kit. After labelling, samples were subjected to LC-MS/MS analysis (RSLCnano liquid chromatography station Ultimate 3000, Thermo Scientific, mass spectrometer- Orbitrap Exploris 240, Thermo Scientific). All samples were analyzed in triplicates. Mass spectrometry data were searched against UP000005640_9606.fasta human database in Proteome Discoverer 2.4 (Thermo Scientific) software with Sequest algorithm. The average abundance of each protein was calculated. The heteroscedastic t-test was used to identify differentially expressed proteins (DEPs) according to clinical factors, and the paired t-test was used to compare protein abundances between study time points. The enrichment of functions and signaling pathways of the identified DEPs was determined using STRING database.
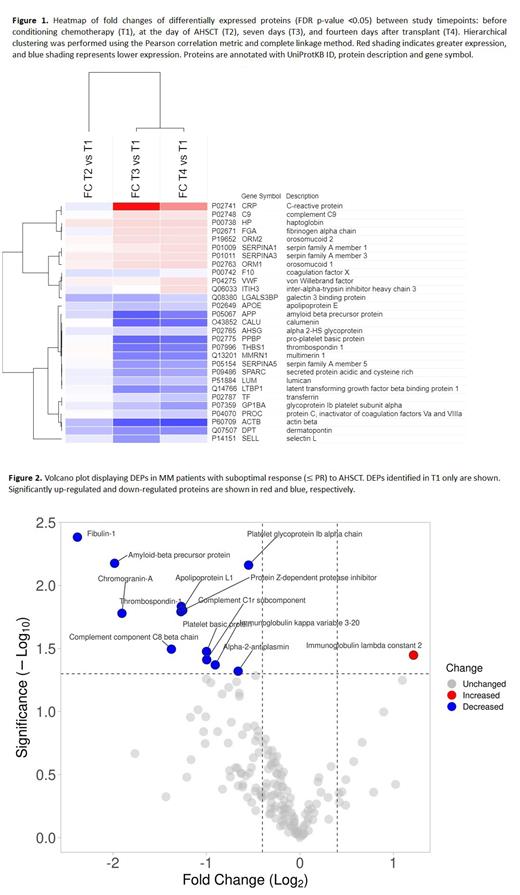
Results: A total of 370 proteins with an average of 178.6 per sample were identified in exosomes derived from MM patients. Using the STRING database, the majority of proteins (207, 55.9%) were found to be localized in extracellular exosomes, confirming that isolated vesicles were exosomes. Proteins present in at least 50% of all samples were included for further analyses. When comparing time points with the application of correction for multiple comparisons, in T2 compared to T1, two downregulated DEPs were found. In T3 compared to T1, 21 DEPs were found, five up-regulated and 16 down-regulated. In T4 compared to T1, 21 DEPs were identified, seven were up-regulated, and 14 were down-regulated (Figure 1). In functional enrichment analysis, among the biological processes with the most involved DEPs between time points were response to stress, defense response, cell adhesion, regulation of stress response, and inflammatory response.
In patients with suboptimal response to AHSCT (≤ PR), at T1 we found 13 DEPs, one up-regulated and 12 downregulated (Figure 2), that were highly enriched in genes related to platelet activation, innate immune system, platelet degranulation, post-translational protein phosphorylation and regulation of insulin-like growth factor.
Conclusions: Our findings highlight the significant alterations in serum exosomal protein content during AHSCT and suggest its potential utility in risk-benefit stratification and optimizing treatment decisions in MM patients undergoing AHSCT.
Disclosures
Wierzbowska:Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Honoraria; Celgene/BMS: Honoraria; JazzPharmaceuticals/swixx: Honoraria; Novartis: Honoraria; Gilead: Honoraria; Pfizer: Honoraria; Servier: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal